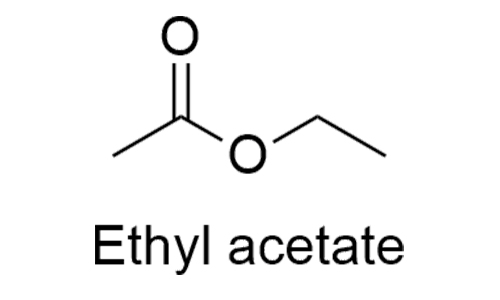
Ethyl Acetate is a stable, colorless and inflammable liquid with pleasant odour. It is used widely in formulating gravure & printing inks, adhesives and lacquers. It is also used extensively as cellulose nitrate solvent in the manufacturing of products such as artificial and patent leathers, inks, cement, and photographic films and linoleum. Ethyl Acetate is also used in the formulation of products like candy glaze, cleaning fluids, flavors and spirit varnish.
Ethyl acetate is used primarily as a solvent and diluent, being favored because of its low cost, low toxicity, and agreeable odor. For example, it is commonly used to clean circuit boards and in some nail varnish removers
Ethyl acetate is a widely used solvent, especially for paints, varnishes, lacquers, cleaning mixtures, and perfumes. Like last week’s MOTW, dichloromethane, it is used as a solvent for decaffeinating coffee beans. In the lab, ethyl acetate is a common solvent for column and thin-layer chromatography
How is it produced:
The main method to manufacture ethyl acetate involves the esterification of ethanol with acetic acid although some is produced by the catalytic condensation of acetaldehyde with alkoxides.
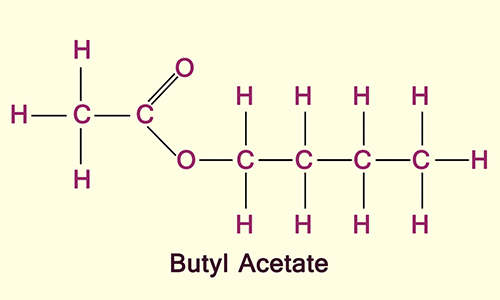
Butyl Acetate
1) as an odor enhancer in perfumes; in the synthesis of synthetics flavors, pharmaceuticals, and other organic compounds;
2) as a thinner for nail enamels and a solvent for leather finishes;
3) as a gasoline additive.
Butyl acetate is made by reacting acetic acid (CH3COOH) with the appropriate butyl alcohol.
The primary use of butyl acetate is as a solvent in the production of lacquers and paints; photographic film; resins; and coatings for furniture, fixtures, containers, and automobiles. Since the 1990s, these compounds have been substituted for other solvents that are considered hazardous environmental pollutants.
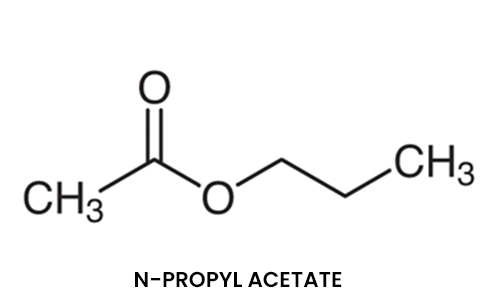
N-Propyl Acetate
N-Propyl Acetate is a common solvent. This clear, colorless liquid is known by its characteristic odour of pears. Due to this fact, it is commonly used as a flavoring additive.
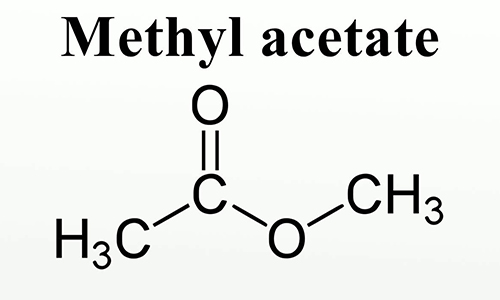
Methyl Acetate
Methyl acetate appears as a clear colorless liquid with a fragrant odor. Moderately toxic. Flash point 14°F. Vapors heavier than air.
Methyl acetate is an acetate ester resulting from the formal condensation of acetic acid with methanol. A low-boiling (57 ℃) colourless, flammable liquid, it is used as a solvent for many resins and oils. It has a role as a polar aprotic solvent, a fragrance and an EC 3.4.19.3 (pyroglutamyl-peptidase I) inhibitor. It is an acetate ester, a methyl ester and a volatile organic compound.
Conventional processes for the hydrolysis of methyl acetate use a fixed-bed reactor followed by a complex arrangement of several distillation/extraction columns. The conversion is limited by unfavourable equilibrium (equilibrium constant 0.14–0.2) and a large amount of unconverted methyl acetate has to be separated and recycled. A schematic diagram of a typical conventional process is given in Figure 6. The reaction is carried out in a fixed-bed reactor and the product stream contains all four components. Four additional columns are required to separate methanol and acetic acid streams and recycle unconverted methyl acetate, along with methanol, to the reactor.